Chapter III: Regulation of adenylate cyclase by the fructose phosphotransferase system
The transport of PTS-sugars other than glucose can affect the phosphorylation of Enzyme IIAGlc simply by generating competition for phosphorylation between the phosphotransfer proteins. Such competition indirectly regulates adenylate cyclase activity. It occurred when enhanced transport of the PTS-sugar fructose takes place (Crasnier-Mednansky M, 1997).
The sequential transfer of phosphate from PEP to fructose does not involve, unlike other PTS, HPr but the diphosphoryl transfer protein FruB (Kornberg HL, 2001), which was renamed Enzyme IIAMHFru, in accordance with the proposed uniform nomenclature for PTS proteins (Saier MH Jr, 1992).
In fruR mutant strains lacking The fructose repressor FruR (also known as Cra), Enzyme IIAGlc is largely unphosphorylated in cells grown in fructose-containing medium. Under these conditions, the fructose operon (fruBKA) is over-expressed thus leading to competition for phosphorylation between Enzyme IIAMHFru and HPr.
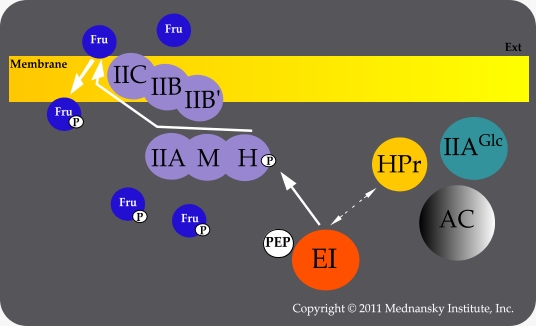
Competition for phosphorylation by Enzyme I (EI) between Enzyme IIAMHFru and HPr
Because of the competition between the phosphoproteins, phosphorylation of Enzyme IIAGlc by HPr is limited; as a consequence activation of adenylate cyclase by phosphorylated Enzyme IIAGlc is impaired. Therefore fruR mutant strains grown on fructose exhibit cAMP levels that are much lower than those in wild-type strains grown on fructose. With carbon sources other than fructose, cAMP levels of fruR and wild-type strains are comparable (Crasnier-Mednansky M, 1997)1. One of the physiological consequences of Enzyme IIAGlc unphosphorylation is that fructose can substitute for glucose in producing diauxie but only in fruR strains, Diauxie Revisited: The Case of Fructose.
Competition for phosphorylation between PTS constituents appears to be a crucial factor for adenylate cyclase regulation, as exemplified by the study of fruR mutant strains. Such competition is also likely to occur when transport of several PTS-sugars takes place. Limited phosphoryl flux may possibly be a constituent of the winner-take-all behavior, as revealed by theoretical studies of metabolic switching in the PTS (Thattai M, 2003).
In the case of a non-PTS carbon source, for example glucose-6-phosphate, the regulation of adenylate cyclase activity is more difficult to apprehend, as further explained in the next chapter. Indeed glucose-6-phosphate transport and/or metabolism is not known to involve PTS proteins, especially Enzyme IIAGlc.
1In an article titled,
Correlation between growth rates, EIIA phosphorylation, and intracellular cAMP levels in
Escherichia coli
K-12, Bettenbrock K, 2007 improperly reported, by referring to Crasnier-Mednansky M, 1997, that lack of HPr enhances cAMP production in fructose-grown E. coli.
This is erroneous as lack of HPr results in a low production of cAMP in
fructose-grown E. coli.
In an article titled, Contributions of SpoT Hydrolase, SpoT Synthetase, and RelA Synthetase to Carbon Source Diauxic Growth Transitions in
Escherichia coli, Fernández-Coll L, 2018, erroneously reported, by referring to Crasnier-Mednansky M, 1997,
that FruR has been proposed to indirectly modify cAMP levels by controlling phosphorylation of EIIB (Enzyme IICBGlc). Enzyme IICBGlc is not involved
in the FruR effect described in Crasnier-Mednansky M, 1997.
The authors also stated, FruR acts independently of CRP, it does not.