CRP-dependent activation of adenylate cyclase
In crp mutant strains of Escherichia coli, lacking the catabolite gene activator protein (CRP also known as CAP), cAMP synthesis is substantially increased as compared to wild type strains (Potter K, 1974; Fraser AD, 1978).
Overproduction of cAMP in crp strains can be visualized on MacConkey agar plate due to cAMP extrusion from the cells. This extrusion can be assessed by using an indicator E. coli cyaA mutant strain that develops red colonies on maltose MacConkey agar, when supplemented with minimal level of cAMP. A culture drop of the strain to be analyzed for cAMP extrusion is plated on a lawn of the indicator strain. After an overnight incubation, a red halo develops around a culture drop of a crp strain. The width of the halo is proportional to the amount of cAMP released. This method of visualization was used to screen E. coli mutant strains impaired in cAMP production (Crasnier M, 1990).
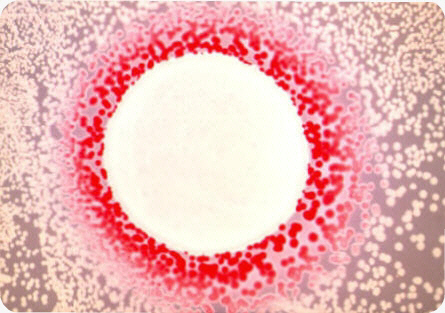
Extrusion of cAMP from a crp strain of Escherichia coli as visualized on maltose MacConkey agar plate
It was found that the increase in cAMP synthesis, which resulted in an overproduction of cAMP, depended mainly on the activation of adenylate cyclase by phosphorylated Enzyme IIAGlc. Indeed, in crp crr mutant strains lacking both CRP and Enzyme IIAGlc, the increase in cAMP did not occur (Crasnier M, 1990). It indicated that the phosphorylated form of Enzyme IIAGlc was involved in a CRP-dependent activation of adenylate cyclase.
It was reported by Krin E, 2002 that the percentage of phosphorylated Enzyme IIAGlc is strongly reduced in glucose-grown wild type strains as compared to glucose-grown crp strains, with no significant difference in the amount of total Enzyme IIAGlc, i.e., phosphorylated plus unphosphorylated. This finding however finds a partial explanation in the fact that glucose transport is much slower in crp strains as compared to wild type strains. Therefore, in crp strains, dephosphorylation of Enzyme IIAGlc by Enzyme IICBGlc is less effective leading to an increased concentration of phosphorylated Enzyme IIAGlc. Consequently, adenylate cyclase activation by phosphorylated Enzyme IIAGlc is more effective in crp strains.
In agreement with the proposal that cAMP synthesis in crp strains is mainly dependent on phosphorylated Enzyme IIAGlc, Takahashi H, 1998 reported that Enzyme IIAGlc is largely phosphorylated in crp strains grown in rich medium. Interestingly, the same authors also reported that addition of a wild type strain cellular extract to a crp strain cellular extract stimulated dephosphorylation of Enzyme IIAGlc. Therefore it was concluded that, in wild type strains, proteins whose synthesis is dependent on the CRP-cAMP complex may promote dephosphorylation of Enzyme IIAGlc. Unfortunately these proteins were not identified.